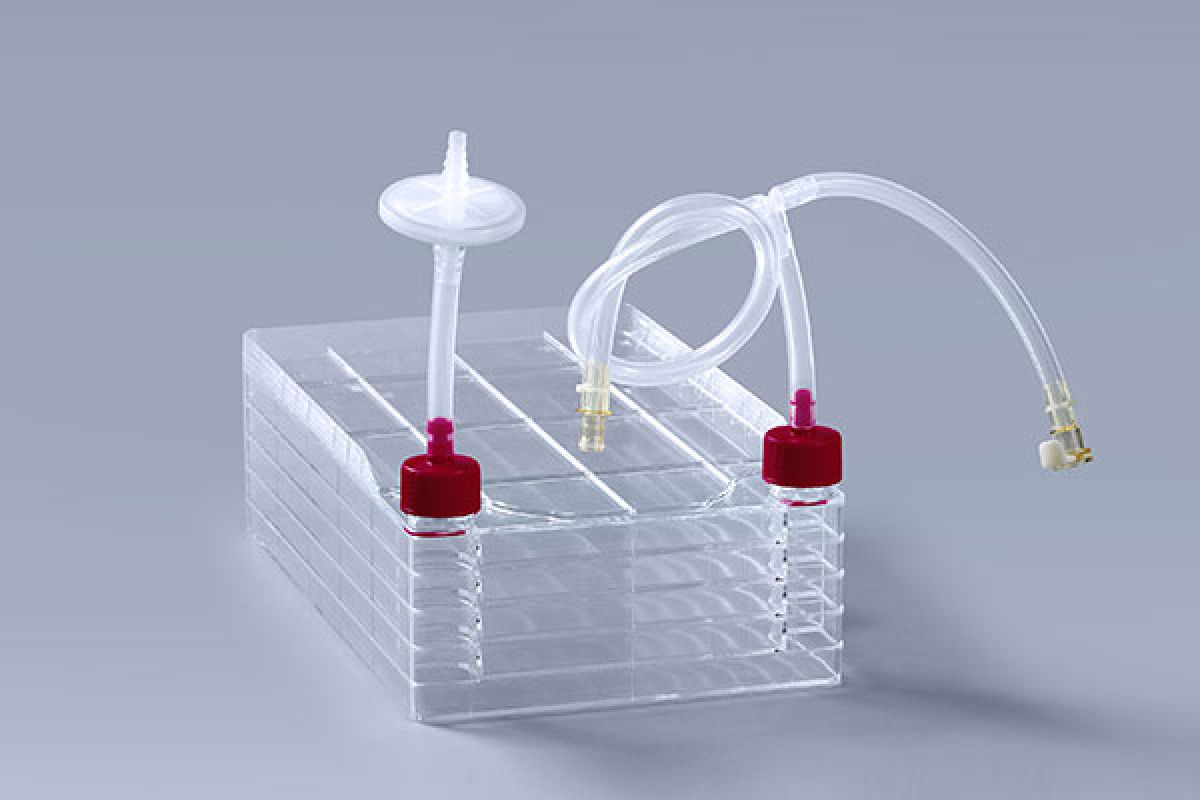
Explore how FDCELL cell factories ensure consistent and scalable cell growth from seed flasks to production scale, supporting reliable bioprocessing and GMP manufacturing.
In biopharmaceutical and vaccine manufacturing, cell culture consistency is the foundation of process reliability and product quality.
As production scales up—from seed flasks to cell factory—cells encounter new physical and chemical environments. Maintaining identical growth conditions during this transition is crucial for ensuring stable cell performance and reproducible yields.
The cell factory, with its multilayer structure and standardized culture surfaces, has become the preferred platform for achieving controlled, scalable, and consistent cell expansion.
1. Maintaining Environmental Continuity Across Scales
When cells are transferred from seed flasks to larger systems, changes in gas exchange, nutrient diffusion, and surface-to-volume ratio can lead to variations in cell behavior.
To minimize these effects, several control points are critical:
Gas Exchange:Uniform CO₂ and O₂ distribution ensures optimal metabolic activity. Cell factories with integrated vent filters and optimized air pathways maintain stable gas flow across all layers.
Temperature and pH Stability:Gradual adaptation to identical incubator conditions prevents stress-induced variability during scale-up.
Surface Uniformity:The TC-treated surfaces of cell factory replicate the same microenvironment as seed flasks, supporting consistent cell attachment and proliferation.
2. Nutrient Management and Medium Flow
In multilayer culture systems, even nutrient distribution is essential to maintain cell health.
Modern cell factories are designed with precision-molded layers to promote consistent medium flow and reduce stagnation zones.
When connected to automated filling and harvest systems, these platforms enable precise control over media exchange cycles—preventing local depletion and supporting reproducible cell growth kinetics.
3. Process Control in Closed Systems
Open transfers between vessels increase contamination risks and process variability.
Integrating cell factory system into a closed-loop culture system with sterile tubing, connectors, and pumps allows for aseptic operations from inoculation to harvest.
This approach not only enhances biosafety but also improves batch-to-batch consistency—especially in GMP-compliant viral vector or vaccine manufacturing.
4. Monitoring and Documentation
Consistency is not only about hardware—it’s also about data traceability.
Modern cell culture workflows pair cell factories with automated monitoring systems for temperature, CO₂ concentration, and growth kinetics.
By collecting and analyzing process data, manufacturers can identify subtle deviations early and maintain high reproducibility between production runs.
5. Design and Material Considerations
High-quality polystyrene (PS) and precise TC treatment ensure each cell factory layer provides identical attachment properties.
FDCELL’s cell factory system uses medical-grade PS materials and is produced in C-grade cleanrooms, with strict ISO-certified quality control—guaranteeing surface uniformity, sterility, and batch reliability.
Conclusion
From seed flask to large-scale cell factory, achieving consistent cell growth is a multidisciplinary challenge involving material science, engineering, and process control.
By focusing on key parameters—gas exchange, surface uniformity, nutrient flow, and aseptic integration—manufacturers can ensure smooth scale-up and reproducible results.
With its standardized design, multilayer scalability, and compatibility with automated closed systems, the cell factory stands as an indispensable tool for reliable, high-efficiency biomanufacturing.